IXCHIQ™ DEMONSTRATED A GENERALLY WELL-TOLERATED SAFETY PROFILE
The safety and tolerability profile of IXCHIQ™ was evaluated from 3 randomized, multicenter clinical studies.1*
The IXCHIQ™ safety data below was collected in 4115 participants from the main VLA1553-301 clinical trial, randomized 3:1 to receive IXCHIQ™ or placebo where 3082 healthy adults (18–88 years of age) received a single dose of IXCHIQ™ and 1033 received a placebo (Phosphate Buffered Saline). Participants were followed-up for safety for 6 months post-vaccination.1
SOLICITED SYSTEMIC AND INJECTION SITE ADVERSE EVENTS WITHIN 10 DAYS AFTER A SINGLE VACCINATION (SAFETY POPULATION IN STUDY VLA1553-301)1
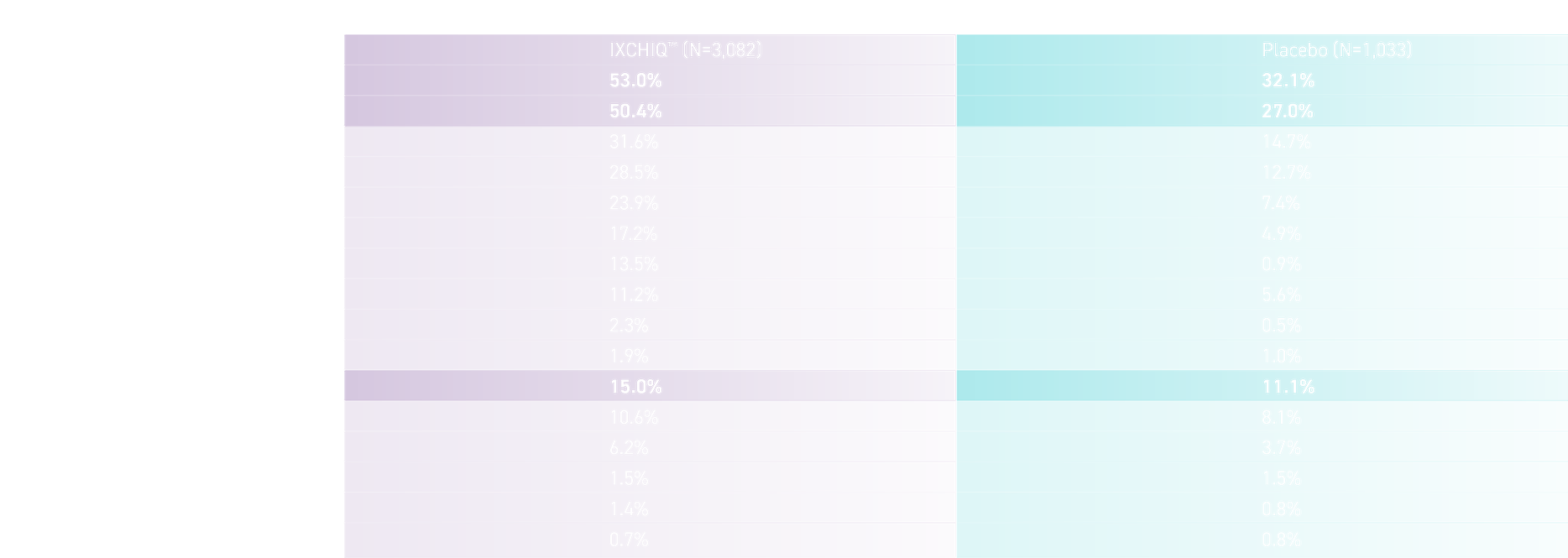
Adapted from the IXCHIQ™ Product Monograph.1
All solicited injection site adverse reactions were graded as mild or moderate in severity, except for a single solicited injection site adverse reaction of pain graded as severe in the IXCHIQ™ group.
Local and systemic adverse reactions resolved with a median duration of 2 days.1
* Studies (VLA1553-301, VLA1553-302, and VLA1553-101) all conducted in North America in healthy adult participants 18 years of age and older. Study VLA1553-101 was done using an earlier formulation and was a supportive dose-escalation Phase I trial of IXCHIQ™ in 120 participants. Study VLA1553-301 was the pivotal double-blind placebo-controlled Phase III trial in 4115 participants who received IXCHIQ™ or placebo (phosphate buffered saline) as a single dose. Study VLA1553-302 was a supportive non-placebo-controlled Phase III trial investigating the consistency of three lots of IXCHIQ™ given as a single dose in 408 participants.
FREQUENCY OF CHIKUNGUNYA-LIKE ADVERSE REACTIONS
- In Study VLA1553-301, participants were monitored for a cluster of symptoms consistent with wild-type chikungunya infection.1
- Chikungunya-like adverse reactions were defined as:1
- Fever (≥38 °C )
- One or more of any of the following: arthralgia or arthritis, myalgia, headache, back pain, rash, lymphadenopathy, or certain neurological, cardiac or ocular symptoms that occurred with an onset within 30 days after vaccination, regardless of whether they occurred simultaneously or not.
- Severe chikungunya-like adverse reactions included symptoms that prevented daily activity and/or required medical intervention.1
- Among Study VLA1553-301 participants, 11.7% in the IXCHIQ™ group (n= 3082) reported chikungunya-like adverse reactions, including 1.6% who reported severe chikungunya-like adverse reactions. 0.6% participants in the placebo group (n=1033) reported chikungunya-like adverse reactions, none of which were severe.
FREQUENCY OF SYMPTOMS AMONG PARTICIPANTS WITH CHIKUNGUNYA-LIKE ADVERSE REACTIONS (STUDY VLA1553-301)1
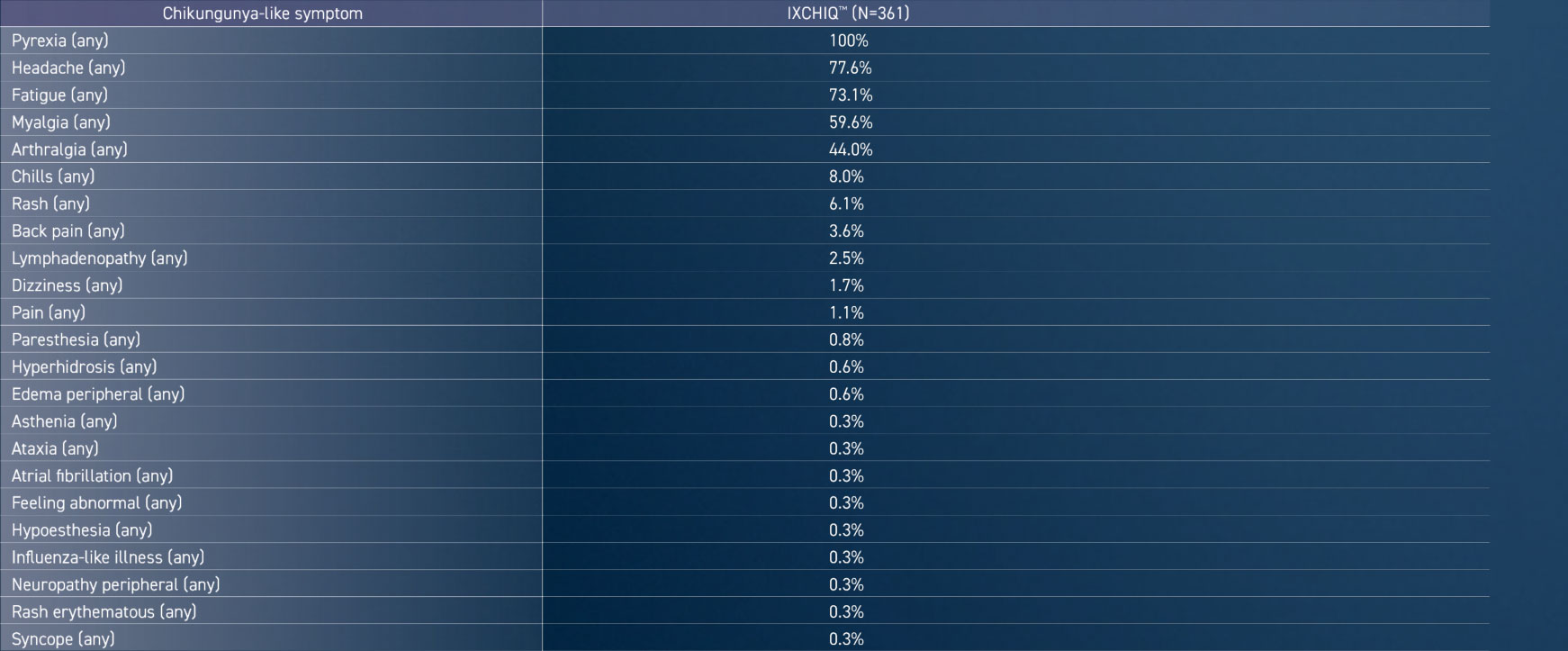
Adapted from the IXCHIQ™ Product Monograph.1
- The median onset of chikungunya-like adverse reactions in IXCHIQ™ recipients was 3.0 days (range 0 to 10 days) after vaccination. The median duration of chikungunya-like adverse reactions in IXCHIQ™ recipients was 4.0 days (range 1 day to at least 6 months) after vaccination.1
- 22 IXCHIQ™ recipients had prolonged chikungunya-like adverse reactions lasting longer than 14 days (median duration 33 days, range 15 days to at least 6 months) and 15 IXCHIQ™ recipients had chikungunya-like adverse reactions lasting longer than 28 days (median duration 94 days, range 29 days to at least 6 months).1
SERIOUS ADVERSE EVENTS
- The proportions of participants who reported at least one serious adverse event within 6 months following administration was 1.5% (46/3082) in the IXCHIQ™ group and 0.8% (8/1033) in the placebo group. Overall, there were two serious adverse events (2/3082 [0.1%]) requiring hospitalization that were considered to be related to IXCHIQ™: 1 event of myalgia, and 1 event of hypovolemic hyponatremia and atrial fibrillation, both events showed full recovery. There were no serious adverse events reported in the placebo group.
DEATHS AND ADVERSE EVENTS LEADING TO WITHDRAWAL FROM STUDY
- Three participants died during Study VLA1553-301 due to events independent of study procedures (coronary artery disease, COVID-19, and anoxic brain injury). None of the deaths was considered as related to IXCHIQ™. Approximately 0.1% of participants who received IXCHIQ™ vs. 0.2% in the placebo group discontinued their trial participation due to adverse events.
For more information on clinical trial adverse reactions, please refer to the IXCHIQ™ Product Monograph.
* Studies (VLA1553-301, VLA1553-302, and VLA1553-101) all conducted in North America in healthy adult participants 18 years of age and older. Study VLA1553-101 was done using an earlier formulation and was a supportive dose-escalation Phase I trial of IXCHIQ™ in 120 participants. Study VLA1553-301 was the pivotal double-blind placebo-controlled Phase III trial in 4115 participants who received IXCHIQ™ or placebo (phosphate buffered saline) as a single dose. Study VLA1553-302 was a supportive non-placebo-controlled Phase III trial investigating the consistency of three lots of IXCHIQ™ given as a single dose in 408 participants.
