CLINICAL DATA: STUDY DESIGN
THE EFFICACY PROFILE OF IXCHIQ™ WAS EVALUATED IN A DOUBLE-BLIND, RANDOMIZED PLACEBO-CONTROLLED PHASE 3 TRIAL1,25
PHASE 3: A PIVOTAL, MULTICENTER, RANDOMIZED, PLACEBO-CONTROLLED, DOUBLE-BLIND TRIAL1,25*
Randomization 3:1


Recruitment was stratified by age (18–64 years and ≥65 years)
PRIMARY IMMUNOGENICITY ENDPOINT1,25
Seroresponse rate (SRR), the surrogate immunogenicity endpoint of efficacy†
- Defined as achieving a virus neutralizing antibody μPRNT50 titer ≥150, which was considered to predict a clinical benefit, for baseline negative participants 28 days post-vaccination
SECONDARY IMMUNOGENICITY ENDPOINT1,25
The immune response as measured by chikungunya virus-specific neutralising antibody titres on Day 8, Day 29, Day 85, and at Month 6 post-vaccination as determined by μPRNT assay.
* Double-blind, randomized placebo controlled trial of 4115 adults (1864 M; 2251 F) aged 18-94. Study subjects received either IXCHIQ™ 1×10E4 TCID50 per 0.5 mL or placebo (phosphate buffered saline) via intramuscular injection as a single dose with 6 month follow up. Healthy adult men and women were enrolled without prior known or suspected chikungunya virus infections and unlikely to become exposed to chikungunya virus during the study. Subjects with chronic illnesses or conditions that were stable and well-controlled on therapy for the past 6 months were eligible to participate in the clinical study. Immuno-compromised subjects were not eligible to participate in the clinical study.
† This is based on nonclinical data from a non-human primate pharmacology study showing that animals treated with immune sera from clinical samples collected in Study VLA1553-101 were protected against mild CHIKV disease induced by challenge with a WT CHIKV strain. Pre-challenge neutralizing antibody titer level about 150 resulted in undetectable virus during 14 days after the challenge.
PRIMARY IMMUNOGENICITY ENDPOINT
Strong SRRs demonstrated in a pivotal study1,25*
IXCHIQ™ INDUCED A SERORESPONSE IN 98.9% OF PARTICIPANTS 28 DAYS AFTER A SINGLE DOSE1,25†‡

Adapted from the IXCHIQ™ Product Monograph and Schneider et al. 1,25
HIGH SRRs WERE OBSERVED AT 28 DAYS POST-VACCINATION AND SUSTAINED AT 180 DAYS POST-VACCINATION1,25‡
28 days post-vaccination
(p<0·0001; 95% CI: 96.7, 99.8)
180 days post-vaccination
(95% CI: 93.1, 98.3)
*Percentage of participants with neutralizing antibody titers above the threshold of ≥150 determined by μPRNT50 titer.
†Success criterion: lower bound of the 95% CI for SRR >70%.
‡Clinical significance unknown.
SRR:seroresponse rate; CI: confidence interval; μPRNT: micro plaque reduction neutralization test.
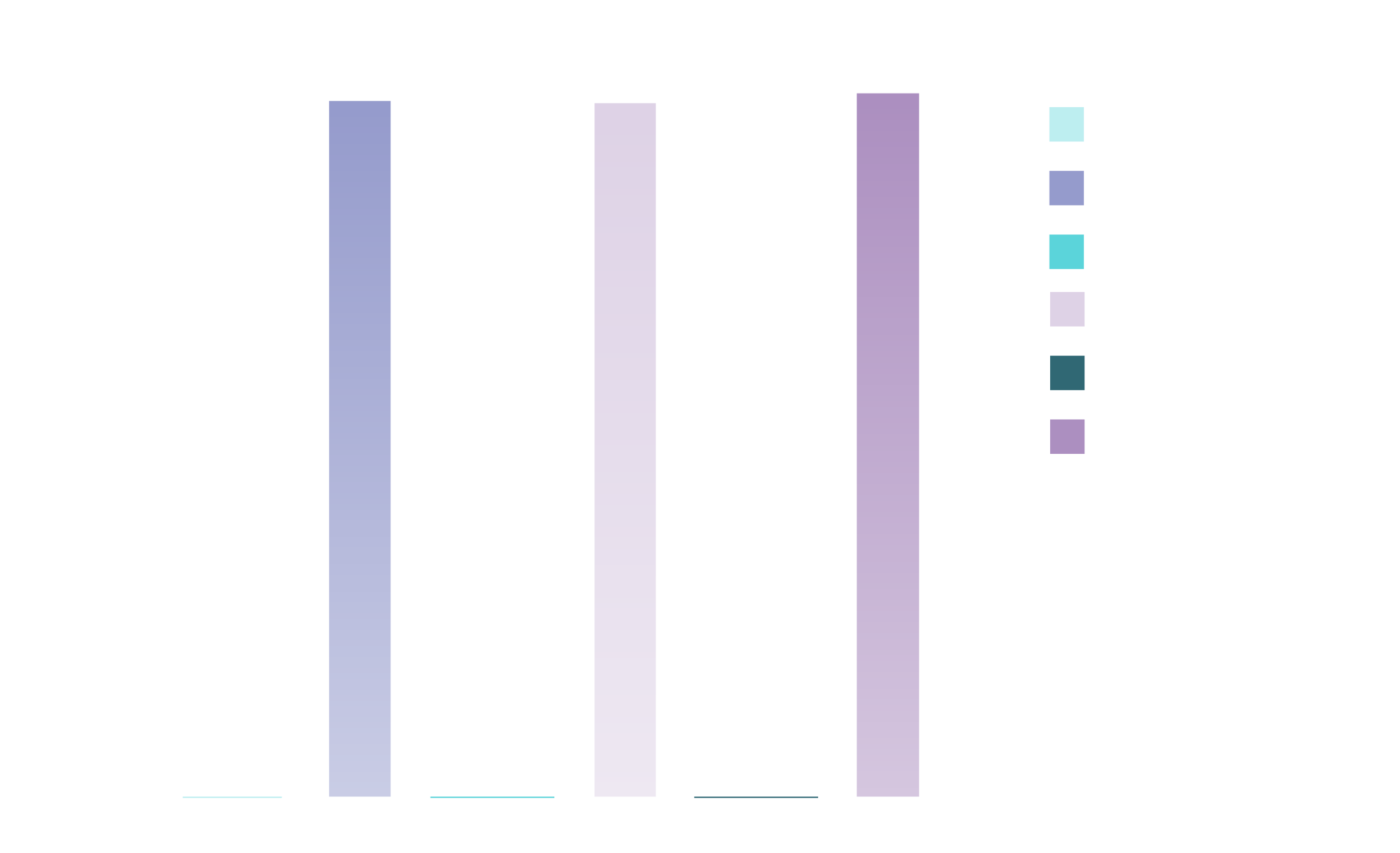
SECONDARY IMMUNOGENICITY ENDPOINT
GMT of chikungunya virus-specific neutralizing antibodies on 28 days and 6 months post-vaccination (PP population)1*†‡


Adapted from the IXCHIQ™ Product Monograph.1
* Double-blind, randomized placebo controlled trial of 4115 adults (1864 M; 2251 F) aged 18—94. Study subjects received either IXCHIQ™ 1×10E4 TCID50 per 0.5 mL or placebo (phosphate buffered saline) via intramuscular injection as a single dose with 6 month follow up. Healthy adult men and women were enrolled without prior known or suspected chikungunya virus infections and unlikely to become exposed to chikungunya virus during the study. Subjects with chronic illnesses or conditions that were stable and well-controlled on therapy for the past 6 months were eligible to participate in the clinical study. Immuno-compromised subjects were not eligible to participate in the clinical study.
† As determined by μPRNT assay.
‡ Clinical significance unknown.
GMT:Geometric Mean Titer; PP: per-protocol; TCID50: 50% tissue culture infectious dose; CI: confidence interval; μPRNT: micro plaque reduction neutralization test.
